Search results for: 'cont'''
-
 T-6543 MEDICAL UNIVERSAL TENSILE TESTING EQUIPMENT
T-6543 MEDICAL UNIVERSAL TENSILE TESTING EQUIPMENTREFERENCE NUMBER: T-6543
MEDICAL UNIVERSAL TENSILE TESTING EQUIPMENT
SHANDONG INDEPENDENTLY RESEARCH AND DEVELOPMENT THIS COMPREHENSIVE TESTING MACHINE FOR SURGICAL MASK & PROTECTIVE CLOTHING, IT IS WIDELY USED IN A VARIETY OF MASK STRENGTH DETECTION PROJECTS.FEATURES:COMPLY WITH NATIONAL STANDARDS, MEDICAL STANDARDS TESTING REQUIREMENTS, AUTOMATIC SOFTWARE CONTROL SYSTEM, MEET THE REQUIREMENTS OF DATA STORAGE, PRINTING, COMPARISON. THE IMPORTED SERVO MOTOR IS EQUIPPED WITH PRECISION SCREW DRIVE SYSTEM TO ENSURE THE STABILITY OF TEST DATA.
TEST STANDARDS:
GB 19082-2009 TECHNICAL REQUIREMENTS FOR DISPOSABLE PROTECTIVE CLOTHING FOR MEDICAL USE (4.5 BREAKING STRENGTH -THE BREAKING STRENGTH OF MATERIALS IN KEY PARTS OF PROTECTIVE CLOTHING SHOULD NOT BE LESS THAN 45N)
(4.6 ELONGATION AT BREAK -THE ELONGATION AT BREAK OF KEY PARTS OF PROTECTIVE CLOTHING SHOULD NOT BE LESS THAN 15%)
SELF-PRIMING FILTER RESPIRATOR FOR RESPIRATORY PROTECTION ARTICLES5.6.2 BREATHING BONNET -BREATHING BONNET SHALL BE SUBJECT TO AXIAL TENSION"DISPOSABLE MASK: 10N FOR 10S" "REPLACEABLE MASK: 50N FOR 10S"
(5.9 HEADBAND -HEADBAND SHOULD BEAR THE TENSION "DISPOSABLE MASK: 10N, 10S" "REPLACEABLE HALF MASK: 50N FOR 10S" "FULL MASK: 150N FOR 10S")
5.10 JOINTS AND CONNECTING PARTS -JOINTS AND CONNECTING PARTS SHALL BE SUBJECT TO AXIAL TENSION
"REPLACEABLE HALF MASK: 50N FOR 10S" "FULL COVER 250N FOR 10S"
GB/T 32610-2016 TECHNICAL SPECIFICATION FOR DAILY PROTECTIVE MASKS
(6.9 BREAKING STRENGTH OF THE MASK BELT AND THE CONNECTION BETWEEN THE MASK BELT ANDTHE MASK BODY ≥ 20N)
(6.10 FASTNESS TO BREATHING BONNET: NO SLIPPAGE, BREAKAGE OR DEFORMATION SHALL OCCUR)
YY/T 0699-2013 DISPOSABLE SURGICAL MASK
(4.4 MASK BELT -THE BREAKING FORCE AT THE CONNECTION POINT BETWEEN EACH MASK BELT AND THE MASK BODY IS NOT LESS THAN 10N)
YY 0469-2011 SURGICAL MASK FOR MEDICAL USE (5.4.2 MASK BELT)
GB/T 3923.1-1997 DETERMINATION OF BREAKING STRENGTH AND ELONGATION AT BREAK OF FABRICS (STRIP METHOD)
DISPOSABLE RUBBER INSPECTION GLOVES (6.3 TENSILE PROPERTIES)
INSTRUMENT TECHNICAL PARAMETERS:
SPECIFICATION: 200N (STANDARD) 50N, 100N, 500N, 1000N (OPTIONAL)
ACCURACY: BETTER THAN 0.5RESOLUTION OF FORCE VALUE: 0.1N
DEFORMATION RESOLUTION: 0.001mm
TEST SPEED: 0.01mm/MIN ~ 2000mm/MIN (STEPLESS SPEED REGULATION)
WIDTH OF SAMPLE: 30mm (STANDARD FIXTURE) 50mm (OPTIONAL FIXTURE)
CLAMPING OF SAMPLES: MANUAL (PNEUMATIC CLAMPING CAN BE CHANGED)
STROKE: 700mm (STANDARD) 400mm, 1000mm (OPTIONAL)
QUANTITY: 1
Learn More -
 T-6545 MASK BACTERIAL FILTRATION EFFICIENCY (BFE) DETECTOR TESTER
T-6545 MASK BACTERIAL FILTRATION EFFICIENCY (BFE) DETECTOR TESTERREFERENCE NUMBER: T-6545
MASK BACTERIAL FILTRATION EFFICIENCY (BFE) DETECTOR TESTER
PRODUCT INTRODUCTION:
MASK BACTERIA FILTRATION EFFICIENCY KEY PERFORMANCE INDICATORS (BFE) DETECTOR TESTER NOT ONLY CONFORMS TO THE REQUIREMENTS OF MEDICAL SURGICAL MASKS TECHNOLOGY YY0469-2011 APPENDIX B IN THE TEST METHOD FOR FILTER EFFICIENCY (BFE) BACTERIA FIRST B. 1.1.1 TEST INSTRUMENT, BUT ALSO CONFORMS TO THE AMERICAN SOCIETY FOR TESTING MATERIAL ASTMF2100, ASTMF2101, THE REQUIREMENTS OF THE EUROPEAN EN14683 STANDARDS, INNOVATIVE IMPROVEMENTS WERE MADE ON THE BASIS OF THIS, WITH DOUBLE PNEUMATIC CONTRAST SAMPLING METHOD AT THE SAME TIME, IMPROVE THE PRECISION OF SAMPLING,IT IS SUITABLE FOR MEASURING AND TESTING DEPARTMENTS, SCIENTIFIC RESEARCH INSTITUTES, MASK MANUFACTURERS AND OTHER RELEVANT DEPARTMENTS TO TEST THE PERFORMANCE OF MASK BACTERIAL FILTRATION EFFICIENCY.
EXECUTION STANDARD:
Q/0212 ZRB003-2015 MEDICAL SURGICAL MASK
BACTERIAL FILTRATION EFFICIENCY (BFE) DETECTOR RELATED INTELLECTUAL PROPERTY:
NO.: ZL200820224142.6 ELECTRIC FLOW CONTROL VALVE
NO.: ZL200820224143.0 GAS CAPACITY
NO.: ZL200920308391.8 AIR FILTRATIONMATERIAL FILTRATION EFFICIENCY DETECTOR
PRODUCT FEATURES:
NEGATIVE PRESSURE TEST SYSTEM TO ENSURE THE SAFETY OF OPERATORS;
BUILT -IN PERISTALTIC PUMP IN NEGATIVE PRESSURE CABINET, A, B TWO -WAY, SIX ANDERSEN; THE PERISTALTIC PUMP FLOW CAN BE SET;
THE FLOW RATE OF NIGHT SPRAY CAN BE SET AND THE ATOMIZATION EFFECT IS GOOD.
EMBEDDED HIGH SPEED INDUSTRIAL MICROCOMPUTER CONTROL; 10.4 INCHES INDUSTRIAL HIGH BRIGHTNESS COLOR TOUCH SCREEN;
USB INTERFACE, SUPPORT U DISK DATA STORAGE;
CABINET BUILT-IN HIGH BRIGHTNESS LIGHTS; BUILT-IN LEAKAGE PROTECTION SWITCH TO PROTECT THE SAFETY OF OPERATORS;
THE INNER LAYER OF THE CABINET IS MADE OF STAINLESS STEEL, THE OUTER LAYER IS SPRAYED WITH PLASTIC AND COLD-ROLLED, AND THE INNER AND OUTER LAYERS ARE INSULATED AND FLAME RETARDANT.
THE FRONT SWITCH TYPE GLASS DOOR IS CONVENIENT FOR THE EXPERIMENTER TO OBSERVE AND OPERATE.
DETACHABLE BRACKET, ADJUSTABLE BRACKET HEIGHT; SUPPORTING AND MOVING DUAL PURPOSE CASTERS.
QUANTITY: 1
Learn More -
 T-6544 SYNTHETIC BLOOD PENETRATION TESTER FOR SURGICAL MASK
T-6544 SYNTHETIC BLOOD PENETRATION TESTER FOR SURGICAL MASKREFERENCE NUMBER: T-6544
SYNTHETIC BLOOD PENETRATION TESTER FOR SURGICAL MASK
TECHNICAL INDICATORS:
1. THE PROTRUDING SAMPLE FIXING DEVICE CAN SIMULATE THE ACTUAL USE STATE OF THE MASK, SET ASIDE THE TEST TARGET AREA WITHOUT DESTROYING THE SAMPLE, AND DISTRIBUTE THE SYNTHETIC BLOOD IN THE TARGET AREA OF THE SAMPLE.
2. THE SPECIAL FIXED-PRESSURE INJECTION DEVICE CAN EJECT A CERTAIN VOLUME OF SYNTHETIC BLOOD IN A CONTROLLED TIME.
3. IT CAN FULLY SIMULATE THE INJECTION SPEED CORRESPONDING TO THE AVERAGE BLOOD PRESSURE OF HUMAN BODY OF 10.6KPA, 16KPA AND 21.3KPA.
4. A FIXED TARGET PLATE IS SET TO BLOCK THE HIGH-PRESSURE EDGE PART OF THE INJECTED LIQUID FLOW AND ONLY ALLOW THE STEADY FLOW PART TO BE SPRAYED ON THE SAMPLE, WHICH INCREASES THE ACCURACY AND REPEATABILITY OF THE LIQUID VELOCITY SPRAYED ON THE SAMPLE.
STANDARDS:
MEDICAL RESPIRATOR TECHNICAL REQUIREMENTS, 5.5 SYNTHETIC BLOOD PENETRATION BARRIER PERFORMANCE
YY/T 0691-2008 INFECTIOUS PATHOGEN PROTECTION EQUIPMENT MEDICAL MASK RESISTANCE TO SYNTHETIC BLOOD PENETRABILITY TEST METHOD (FIXED VOLUME, HORIZONTAL INJECTION)
YY 0469-2011 MEDICAL SURGICAL MASK TECHNOLOGY REQUIRES A BLOOD PENETRATION TEST DEVICE
ISO 22609:2004 INFECTIOUS PATHOGEN PROTECTION EQUIPMENT MEDICAL MASK TEST METHOD FOR RESISTANCE TO SYNTHETIC BLOOD PENETRATION (FIXED VOLUME, HORIZONTAL INJECTION)
ASTM F1862-07 STANDARD TEST METHOD FOR RESISTANCE OF MEDICAL FACE MASKS TO BLOT BY SYNTHETIC BLOOD (HORIZONTAL PROJECTION OF FIXED VOLUME AT A KNOW VELOCITY)
TECHNICAL PARAMETERS:
1. INJECTION DISTANCE: 300mm ~ 305mm ADJUSTABLE
2. NOZZLE DIAMETER: 0.84mm
3. INJECTION SPEED: 450CM/S, 550CM/S, 635CM/S
4. WEIGHT: 35KG
5. POWER SOURCE: AC220V 50HZ
QUANTITY: 1
Learn More -
 T-6547 RESPIRATOR RESISTANCE TESTER FOR FACE MASKS
T-6547 RESPIRATOR RESISTANCE TESTER FOR FACE MASKSREFERENCE NUMBER: T-6547
RESPIRATOR RESISTANCE TESTER FOR FACE MASKS
INTRODUCTION
THE RESPIRATOR RESISTANCE TESTER IS USED TO MEASURE THE INSPIRATORY RESISTANCE AND EXPIRATORY RESISTANCE OF RESPIRATORS AND RESPIRATOR PROTECTORS UNDER SPECIFIED CONDITIONS. IT IS APPLICABLE TO THE NATIONAL LABOR PROTECTION EQUIPMENT INSPECTION INSTITUTIONS, MASK MANUFACTURERS FOR THE GENERAL MASKS, DUST MASKS, MEDICAL MASKS, ANTI-SMOG MASKS PRODUCTS OF THE RELEVANT TESTING AND INSPECTION.
STANDARD
GB 19083-2010 TECHNICAL REQUIREMENTS FOR MEDICAL PROTECTIVE MASKS
GB 2626-2006 RESPIRATOR SELF-SUCTION FILTER RESPIRATOR AGAINST PARTICULATE MATTER
GB/T 32610-2016 TECHNICAL SPECIFICATIONS FOR DAILY PROTECTIVE MASKS
FEATURES:
1. HIGH-DEFINITION LCD DISPLAY.
2. DIGITAL DIFFERENTIAL PRESSURE METER WITH HIGH PRECISION IMPORTED BRAND.
3. HIGH PRECISION IMPORTED BRAND OF DIGITAL FLOWMETER, WITH HIGH FLOW CONTROL ACCURACY FEATURES.
4. THE RESPIRATOR RESISTANCE TESTER CAN SET UP TWO MODES: EXHALATION DETECTION AND INHALATION DETECTION.
5. AUTOMATIC PIPELINE SWITCHING DEVICE OF RESPIRATOR SOLVES THE PROBLEM OF PIPE EXTUBATION AND WRONG CONNECTION WHEN TESTING.
PARAMETER:
1. FLOWMETER RANGE: 0 ~ 100L/MIN, ACCURACY IS 3%
2. DIGITAL PRESSURE DIFFERENCE METER RANGE: 0 ~ 2000PA, ACCURACY: 1PA
3. PUMPING CAPACITY OF AIR PUMP: >100L/MIN
4. OVERALL SIZE: 460 X 1080 X 670mm
5. POWER SOURCE: AC220V 50HZ 650W
6. WEIGHT: 55KG
QUANTITY: 1
Learn More -
 T-6549 CONSTANT TEMPERATURE AND HUMIDITY CHAMBER -FABRIC WATER VAPOR TRANSMISSION RATE
T-6549 CONSTANT TEMPERATURE AND HUMIDITY CHAMBER -FABRIC WATER VAPOR TRANSMISSION RATEREFERENCE NUMBER: T-6549
CONSTANT TEMPERATURE AND HUMIDITY CHAMBER -FABRIC WATER VAPOR TRANSMISSION RATE
TESTING METER (WITH MOISTURE PERMEABLE CUP)
TECHNICAL DESCRIPTION OF THE INSTRUMENT:
IT IS MAINLY USED TO MEASURE THE MOISTURE PERMEABILITY OF ALL KINDS OF FABRICS, INCLUDING PERMEABLE COATED FABRICS. STRUCTURE PRINCIPLE: ADOPT COMPUTER CONTROL, CREATING A CONSTANT TEMPERATURE, AND HUMIDITY TEST ENVIRONMENT, TEST ENVIRONMENT IN THE CONSTANT TEMPERATURE, AND HUMIDITY, MOISTURE VAPOR TRANSMISSION CUP, PLACED 6 SAMPLE INTO THE GLASS AND THE RUBBER GASKET SEAL, THE CONTAINING HYGROSCOPIC AGENT OR WATER SEAL IS SPECIFIED BY THE WET CUP PLACED IN THE FABRIC SAMPLE TEMPERATURE AND HUMIDITY OF THE ENVIRONMENT, THE SEAL MOISTURE VAPOR TRANSMISSION CUP ACCORDING TO A CERTAIN TIME (INCLUDING SAMPLE AND HYGROSCOPIC AGENT OR WATER) TO CALCULATE THE MOISTURE TRANSMISSION QUALITY CHANGE.
TESTING STANDARD:
GB19082-2009 MEDICAL PRIMARY PROTECTIVE CLOTHING
TECHNICAL REQUIREMENTS GUIDELINES FOR THE SELECTION OF YY-T1498-2016 MEDICAL PROTECTIVE CLOTHING
GB/T12704.1 DETERMINATION OF MOISTURE PERMEABILITY OF FABRICS --HYGROSCOPIC METHOD
DETAILED TECHNICAL SPECIFICATIONS AND CONFIGURATION:
TECHNICAL INDICATORS:
1. TEMPERATURE CONTROL RANGE: -40°C ~ 150°C;RESOLUTION; 0.1 °C
2. HUMIDITY CONTROL RANGE: 50%RH ~ 95% RH±5%
3. SPEED RANGE: 2mm ~ 60mm/MIN
4. CONTROL PRECISION: TEMPERATURE <0.1°C; HUMIDITY + / -1% RH OR LESS
5. CYCLIC WIND SPEED: 0.02 ~ 0.5M/S, 0.3 ~ 0.5M/S
6. TIME CONTROL: 1 ~ 9999H
7. MOISTURE PERMEABLE AREA: 2827 mm 2 (DIAMETER IS 60 mm --NATIONAL STANDARD)
8. QUANTITY OF PERMEABLE CUPS: 6 GB;
9. DRYING BOX CONTROL TEMPERATURE: ROOM TEMPERATURE ~ 199 °C
10. TEST TIME: 1 ~ 999H
11. DRYING BOX STUDIO SIZE: 490 X 400 X 215mm
INSTRUMENT CONFIGURATION:
1. ONE MAIN MACHINE
QUANTITY: 1
Learn More -
 T-6550 MEDICAL PROTECTIVE CLOTHING BLOOD SYNTHETIC PENETRABILITY TESTER
T-6550 MEDICAL PROTECTIVE CLOTHING BLOOD SYNTHETIC PENETRABILITY TESTERREFERENCE NUMBER: T-6550
MEDICAL PROTECTIVE CLOTHING BLOOD SYNTHETIC PENETRABILITY TESTER
TECHNICAL SPECIFICATIONS AND CONFIGURATION:
1. THE INSTRUMENT ADOPTS AN AIR SOURCE THAT CAN PROVIDE (0.5 ~ 30±0.1) KPA PRESSURE TO CONTINUOUSLY PRESSURIZED THE SAMPLE, WHICH IS NOT RESTRICTED BY THE SPACE OF THE TEST SITE;
2. THE AIR PRESSURE RANGE CAN BE ADJUSTED FREELY, AND THE ADJUSTMENT RANGE (0.5 ~ 30) KPA;
3. COLOR TOUCH SCREEN DISPLAY AND OPERATION;
4. THE SAMPLE CLAMPING PAD IS PROCESSED WITH IMPORTED SPECIAL ALUMINUM PROFILES, WHICH IS LIGHT IN MATERIAL, CLEAN IN SURFACE AND NEVER RUSTS.
5. THE INSTRUMENT ADOPTS IMPORTED SPECIAL ALUMINUM WIRE DRAWING PANEL, EQUIPPED WITH METAL KEYS, SENSITIVE OPERATION, NOT EASY TO DAMAGE;
6. CLAMPING DEVICE FOR INSTRUMENT SAMPLES, EQUIPPED WITH LOCKING PROTECTION, TO PREVENT THE SYNTHETIC BLOOD FROM SPLASHING AROUND;
7. CLAMPING FORCE IS ACCURATE AND RELIABLE;
8. THE TEST TANK IS EQUIPPED WITH A SPECIAL PLACEMENT DEVICE, WHICH IS CONVENIENT FOR CUSTOMERS TO OPERATE;
9. THE TEST TANK IS PROCESSED WITH SPECIAL 316 STAINLESS STEEL, AND THE TOP IS EQUIPPED WITH A SPECIAL COVER FOR HIGH TRANSPARENT PROTECTION;
10. THE WASHER BELOW THE SAMPLE IS MADE OF HIGH-QUALITY PTFE MATERIAL AFTER SPECIAL PROCESSING;
11. SQUARE METAL BLOCK NET: OPEN SPACE ≥50°%; BENDING ≤5mm AT 30KPA;
12. INSTRUMENT TIME CONTROL ACCURACY ≤01 SECONDS;
13. THE SHELL OF THE INSTRUMENT IS MADE OF HIGH-QUALITY METAL BAKING PAINT, WHICH IS BEAUTIFUL AND GENEROUS.
14. EQUIPPED WITH PRINTER INTERFACE, CONNECTED TO THE PRINTER CAN DIRECTLY PRINT DATA REPORTS.
TECHNICAL PARAMETERS:
1. SAMPLE SIZE: 75mm X 75mm
2. TEST AREA: 28.26 SQUARE CENTIMETERS
3. AIR PRESSURE ADJUSTMENT RANGE (0.5 ~ 30) KPA
4. EXTERNAL SIZE: 500mm X 500mm X 500mm (L X W X H)
5. WEIGHT OF THE INSTRUMENT: 40 KG
6. POWER SUPPLY: AC220V, 50HZ,SCOPE OF SUPPLY: 1. ONE MAIN MACHINE; 2. TWO PTFE GASKETS; 3. TWO ORDINARY WASHERS; 4. ONE METAL BLOCKING NET; 5. A SET OF SPECIAL LOCKING TOOLS; 6. ONE HIGH-QUALITY SILENT AIR PUMP, [NOTE: SAFETY PRODUCTION LICENSE IS NOT INCLUDED]. 7. EQUIPPED WITH PRINTER INTERFACE; 8. ONE SAMPLE TEMPLATE; 9. ONE PRODUCT CERTIFICATE; 10. ONE COPY OF PRODUCT OPERATION MANUAL.
OPERATION INSTRUCTION:
INSERT THE AIR PUMP OUTPUT PIPE INTO THE OIL AND WATER SEPARATOR OF THE INSTRUMENT AS REQUIRED (PRESSURE 0.1-0.3MPA).
AFTER SWITCHING ON THE POWER, THE STARTUP INTERFACE (NAME OF THE DEVICE) IS DISPLAYED.
PRESS THE "ENTER" BUTTON TO ENTER THE TEST INTERFACE, AS SHOWN IN THE FOLLOWING FIGURE:
CLICK "SETTING” BUTTON, GET INTO PARAMETER SETTING SCREEN, AS BELOW THIS SCREEN CAN SET THE SYSTEM TIME, DATE AND TESTING TIME, AFTER SETTING, CLICK "SAVE” BUTTON TO KEEP THE SETTING DATA. (NOTE: IT REQUIRES 5 MINUTES IN THE STANDARD)
QUANTITY: 1
Learn More -
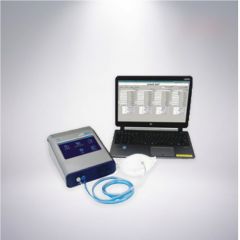 T-6552 MASK AIR TIGHTNESS TESTER
T-6552 MASK AIR TIGHTNESS TESTERREFERENCE NUMBER: T-6552
MASK AIR TIGHTNESS TESTER
MASK AIR TIGHTNESS TESTER
HOW TO CHOOSE A HIGH-QUALITY MASK NOT ONLY DEPENDS ON THE FILTERING EFFICIENCY OF THEMASK, BUT ALSO TO CONFIRM WHETHER THE MASK IS COMPLETELY IN CONTACT WITH THE FACE, OTHERWISE AEROSOL PARTICLES SUCH AS GERMS THAT HAVE NOT BEEN FILTERED BY THE MASK WILL BE INHALED FROM THE INADEQUATE ADHESION. THEREFORE, IT IS VERY IMPORTANT TO CHECK THE WEARING STATUS OF THE MASK. THE EUROPEAN AND AMERICAN COUNTRIES HAVE LISTED THE APPLICATION TEST OF THE MASK AS A STRONG INSPECTION ITEM.
FOR EXAMPLE, THE N95 MASK IS ONE OF 9 TYPES OF PARTICULATE PROTECTIVE MASKS CERTIFIED BY NIOSH (NATIONAL INSTITUTE OF OCCUPATIONAL SAFETY AND HEALTH). "N" INDICATES OIL RESISTANCE."95" INDICATES THAT THE PARTICLE CONCENTRATION IN THE MASK IS MORE THAN 95% LOWER THAN THAT OF THE PARTICLES OUTSIDE THE MASK WHEN EXPOSED TO THE SPECIFIED NUMBER OF SPECIAL TEST PARTICLES. N95 IS NOT A SPECIFIC PRODUCT NAME, AS LONG AS IT MEETS THE N95 STANDARD AND PASSES THE NIOSH REVIEW, IT CAN BE CALLED "N95 MASK".
N95 TYPE MASKS, IN ADDITION TO THE FILTERING EFFICIENCY OF THE MASKS, THE CLOSENESS OF THE MASKS TO THE FACE IS ONE OF THE IMPORTANT FACTORS THAT DETERMINE THE EFFECTIVENESS OF THE USE OF THE MASKS. THE SUITABILITY OF DIFFERENT TYPES OF MASKS FOR THE HUMAN FACE VARIES GREATLY.
THEREFORE, BEFORE USING A MASK, THE SUITABILITY OF THE MASK SHOULD BE CHECKED FIRST. DURING THETIGHTNESS TEST OF THE WEARER'S FACE, ENSURE THAT AIR CAN PASS IN AND OUT THROUGH THE MASK WHEN IT IS CLOSE TO THE EDGE OF THE FACE. THE MASK TIGHTNESS TESTER CAN QUICKLY COMPLETE THE TIGHTNESS TEST OF RESPIRATORS SUCH AS MASKS TO ENSURE THAT IT PROVIDES GOOD PROTECTION PERFORMANCE. IT CONFORMS TO THE CHINESE RESPIRATOR STANDARD GB2626-2019 STANDARD, OSHA / CSA STANDARD AND CHINA GENERAL ADMINISTRATION OF QUALITY SUPERVISION, INSPECTION AND QUARANTINE "GB 19083-2010 TECHNICAL REQUIREMENTS FOR MEDICAL PROTECTIVE MASKS" JOINTLY ISSUED BY THE CHINA NATIONAL STANDARDIZATION ADMINISTRATION COMMITTEE.
ADHESION (SUITABILITY TEST): THE MASK DESIGN SHOULD PROVIDE GOOD ADHESION. THE OVERALL FIT FACTOR OF THE MASK SHOULD NOT BE LESS THAN 100. THIS TEST THE REQUIREMENTS WERE FORMALLY IMPLEMENTED ON AUGUST 1, 2011.
Learn MoreMASK TIGHTNESS TESTER USES CNC TECHNOLOGY, SUITABLE FOR 100 / 99 / P3 / HEPASERIES MASKS DISPOSABLE FILTER MASK TIGHTNESS TEST (INCLUDING DISPOSABLE DUST MASKS SUCH ASN95 / N90 / KN95), GAS MASKS / BREATHING MASKS, ADHESIVENESS TEST OF HALF-MASK AND FULL FACE MASK, INDEPENDENT OR COMPUTER CONTROL, FIVE LANGUAGES SWITCH DISPLAY, EQUIPPED WITH MULTIPLE COMMUNICATION INTERFACES (USB, ETHERNET), WIFI CAN ALSO BE ENABLED, ONE COMPUTERCAN CONTROL FOUR INSTRUMENTS AT THE SAME TIME.PARAMETER:CONCENTRATION RANGE: 0 ~ 100,000 / CM3GRAIN DIAMETER: 0.02 ~ 1.0^MFLOW: SAMPLING FLOW: 100CM3 / MINTOTAL FLOW: 700CM3 / MINCLOSENESS COEFFICIENT TEST: DIRECT TEST (COUT/ CIN)LIQUOR: 99.5% + ISOPROPANOL (ANALYTICAL GRADE)DISPLAY: 7INCH TRUE COLOR TOUCH SCREENCOMMUNICATION INTERFACE: USB X 3 (HOST X 2,DEVICE X 1) ETHERNET INTERFACE X1CONNECTION PORT: ENVIRONMENT PORT, SAMPLING PORTWIFI: EQUIPPEDLANGUAGES: ENGLISH, FRENCH, SPANISH, PORTUGUESE, CHINESEFLOW CONTROL: SENSOR CONTROLPC CONTROLLABLE OPERATION: ONE COMPUTER CAN CONTROL 4 INSTRUMENTS AT THE SAME TIMEDATA OUTPUT FORMAT: MICROSOFT EXCELWORKING TEMPERATURE: 15 ~ 35 °CPOWER SOURCE: AC 110 —240V 50 / 60HZAPPEARANCE SIZE: 208 X 117 X 262MMWEIGHT: 2.1KGACCESSORIES: ALCOHOL REAGENT BOTTLE, PROTECTIVE CAP, REAGENT STICK, ZERO-COUNT FILTER, STRAINER, SAMPLING TUBE, INSTRUCTION MANUAL, AC ADAPTER, TOUCH SCREEN PEN, OPTIONAL COMPUTER.OPTIONAL: TEST KIT FOR TIGHTNESS COEFFICIENTIN ORDER TO PREVENT INFECTION AT THE MEDICAL SITE AND DURING LABOR WORK, WORKERS MUST BE PROTECTED FROM INHALABLE HAZARDOUS SUBSTANCES IN THE WORKPLACE, AND RESPIRATORS SUCH AS MASKS MUST BE WORN DURING WORK.SELECT A RESPIRATOR, SUCH AS A MASK, BASED ON YOUR FACIAL FEATURES, AND EVALUATE THE TIGHTNESS BETWEEN THE RESPIRATOR, SUCH AS A MASK AND THE FACE, TO CHECK WHETHER THERE ARE GAPS OR LEAKS THAT PUT WORKERS AT RISK. THE MASK TIGHTNESS TESTER CAN QUICKLY COMPLETE THE TIGHTNESS TEST OF RESPIRATORS SUCH AS MASKS TO ENSURE THAT IT PROVIDES GOOD PROTECTIVE PERFORMANCE. SECURITY EXPERTS WILL ALSO DEVELOP PROTECTION SCHEMES AND STANDARD REGULATIONS BASED ON THE RESULTS OF THE TIGHTNESS TEST. WIDELY USED IN HOSPITALS, MANUFACTURING PLANTS, PRODUCTION SITES, FIREFIGHTING WORKPLACES AND OTHER OFFICIAL TESTING AGENCIES.MASK TIGHTNESS TESTER FEATURES:• QUANTITATIVE TIGHTNESS TEST FOR RESPIRATORS SUCH AS MASKS (QNFT)• APPLICABLE TO 100/99 / P3 / HEPA SERIES MASK DISPOSABLE FILTER MASK TIGHTNESS TEST (INCLUDING N95 / N90 / KN95 AND OTHER DISPOSABLE DUST MASKS)• ADHESION TEST OF HALF MASK AND FULL FACE MASK• GAS MASK TIGHTNESS TEST• PAPR MASK TIGHTNESS TEST• SCBA RESPIRATOR MASK TIGHTNESS TEST• 7INCH TRUE COLOR TOUCH SCREEN• INDEPENDENT OR COMPUTER CONTROLLED• USING CNC TECHNOLOGY• ENGLISH, FRENCH, SPANISH, PORTUGUESE, CHINESE LANGUAGE SWITCHING DISPLAY• COMPLIES WITH US OSHA STANDARDS, CANADIAN STANDARDS ASSOCIATION (CSA) GUIDELINES, INCLUDING N95• EQUIPPED WITH A VARIETY OF COMMUNICATION INTERFACES (USB, ETHERNET), AND CAN ALSO ENABLE WIFI• ONE COMPUTER CAN CONTROL FOUR INSTRUMENTS AT THE SAME TIMEQUANTITY: 1 -
 T-6581 MEDICAL DISPOSABLE PROTECTIVE CLOTHING SUIT
T-6581 MEDICAL DISPOSABLE PROTECTIVE CLOTHING SUITREFERENCE NUMBER: T-6581
MEDICAL DISPOSABLE PROTECTIVE CLOTHING SUIT
MEDICAL DISPOSABLE PROTECTIVE CLOTHING SUIT
MEETS GB19082-2009
EN14126 TESTING STANDARD
CAPACITY 100,000 PIECES/DAY(CAPACITY CAN BE ENLARGEDACCORDING TO THE ORDER)
PRODUCTION CAPACITY IS EXPANDING
LARGE QUANTITY IS PREFERRED
THE PAYMENT METHOD IS 100% IN ADVANCE
NOTE: IN THE MASS PRODUCTION OF PROTECTIVE CLOTHING, THE GOVERNMENT, HOSPITALS, AND DISEASE CONTROL CENTERS HAVE PRIORITY IN PURCHASING
MATERIAL: MADE OF 65GWHITE COMPOSITE FILM + 30G NONWOVEN FABRIC + 25G FILM + 3G GLUE + 2.0CM EVA 0.12MMSEALED LINE, TWO PARTS OF PROTECTIVE CLOTHING, HOODEDTOTAL WEIGHT OF CLOTHING IS ABOUT 330-350 G
ONLY HOSPITAL ICU REQUIRED STERILE TYPE, ALL OTHER PLACESREQUIRED NON-STERILE TYPEPACKAGE: MEDICAL PROTECTIVE CLOTHING
CARTON OUTER BOX SIZE: 560 X360 X400mm
SINGLE BOX WEIGHT: 13.5 KG(VOLUME WEIGHT:16.5 KG)45 PIECES PER BOX
MINIMUM ORDER QUANTITY: 10,000 PIECES
Learn More -
 J-2811 AIRBORNE PARTICLE COUNTER
J-2811 AIRBORNE PARTICLE COUNTERAIRBORNE PARTICLE COUNTER
PORTABLE PARTICLE COUNTER WITH 0.10 MICRON SENSITITIVITY AND 1.0 CFM (28.3 LPM) FLOW RATE USING LONG LIFE LASER DIODE TECHNOLOGY.
DESIGNED TO OPERATE IN ISO CLASS 1 TO CLASS 7 CLEANROOMS WITHOUT CONCERNS OF IT DEGRADING ITS ENVIRONMENT OR EXCEEDING CONCENTRATION LIMITS
USES AN OPTICS AND PHOTODIODE SYSTEM DESIGNED TO REDUCE OR ELIMINATE THE TEMPERATURE ISSUES ASSOCIATED WITH HIGH POWER LASER DIODE SENSORS
WITH THERMALLY CONTROLLED EXHAUST SYSTEM AND HEPA FILTER OPTION TO PREVENT ANY EXTERNAL CONTAMINATIONFEATURES
0.1- 1.0 µm SENSITIVITY
1.0 CFM (28.3 LPM)
UP TO 8 CHANNELS OF SIMULTANEOUS COUNT DATA
21 CFR PART 11 COMPLIANT SECURE USB FLASH TRANSFER
LASER DIODE SENSOR TECHNOLOGY
5.7 INCH COLOR TOUCH SCREEN DISPLAY
THERMAL PRINTER
STAINLESS STEEL CONSTRUCTION
ETHERNET/RS485/MODBUS, 4X 4-20MA ANALOG OUTPUTS
3,000 RECORD DATA STORAGE
50-RECORD CONFIGURABLE RECIPE DATABASE
Report wizard: ISO-14644-1, EU GMP Annex 1, FS-209ESTANDARD CHANNEL SIZES: 0.10, 0.15, 0.2, 0.25, 0.3, 0.5, 0.7, 1.0 µm
INCLUDES
OPERATING MANUAL ON CD; ISOKINETIC SAMPLE PROBE WITH TRIPOD AND TUBING; PURGE FILTER; POWER CABLE AND SPARE FUSE; LMS XCHANGE AND EXPRESS SOFTWARE; PRINTER; PRINTER PAPERQUANTITY: 1
Learn More -
 T-6560 NON-STERILIZATION AND STERILIZATION PROTECTIVE CLOTHING
T-6560 NON-STERILIZATION AND STERILIZATION PROTECTIVE CLOTHINGAVAILABILITY: NON-STERILE 740CTNS/ 29600PCS, STERILE 720CTNS/ 14400PCS
STERILE TYPE: 1PC PER BAG, 20 BAGS PER CARTON
NON-STERILE TYPE: 1PC PER BAG, 40 BAGS PER CARTON
WEIGHT AND DIMENSIONS OF CARTONS:
STERILE TYPE: 510 X 370 X 380mm, WEIGHT IS AROUND 6.5KG
NON-STERILE TYPE: CARTON SIZE: 560 X 350 X 350mm, WEIGHT IS AROUND 10.4KG
NO PALLET
CARTONS IN A 20 FOOT CONTAINER:
STERILE TYPE: 720 CARTONS/20'GP
NON-STERILE TYPE: 740 CARTONS/20'GP
STANDARD MATERIAL OF PROTECTIVE CLOTHINGNON-STERILIZATION PROTECTIVE CLOTHING
MODEL: RS-PTCL-M175 OR RS-PTCL-L180 OR RS-PTCL-XL185
ITEM 001
QUANTITY: 1000 PCS
ITEM 002
QUANTITY: 10000 PCSSTERILIZATION PROTECTIVE CLOTHING
Learn More
MODEL: RS-PTCL-M175-S OR RS-PTCL-L180-S OR RS-PTCL-XL185-S
ITEM 003
QUANTITY: 1000 PCS
ITEM 004
QUANTITY: 10000 PCS
