Search results for: 'pro+4+pro'
-
 J-3392 AUTOMATIC 3-PLEAT HEADBAND FISH-STYLE FACE MASK MACHINE -CAPACITY: 45 TO 50 PIECES PER MINUTE -INCLUDES PRINTING ON MASKS -INCLUDES NOSE FOAM INSERTION (CLOSED CELL FOAM)J-3392 AUTOMATIC 3-PLEAT HEADBAND FISH-STYLE FACE MASK MACHINE -CAPACITY: 45 TO 50 PIECES PER MINUTE -INCLUDES PRINTING ON MASKS -INCLUDES NOSE FOAM INSERTION (CLOSED CELL FOAM) REQUIRES 1 TO 2 OPERATORS WITH PLC CONTROLS WEIGHT: 1600 KGS VOLTAGE: 220 VOLTS POWER: 6 KW ULTRASONIC FREQUENCY: 20 kHz GAS PRESSURE: 6 KG PER cm2 QUANTITY: 1 Learn More
J-3392 AUTOMATIC 3-PLEAT HEADBAND FISH-STYLE FACE MASK MACHINE -CAPACITY: 45 TO 50 PIECES PER MINUTE -INCLUDES PRINTING ON MASKS -INCLUDES NOSE FOAM INSERTION (CLOSED CELL FOAM)J-3392 AUTOMATIC 3-PLEAT HEADBAND FISH-STYLE FACE MASK MACHINE -CAPACITY: 45 TO 50 PIECES PER MINUTE -INCLUDES PRINTING ON MASKS -INCLUDES NOSE FOAM INSERTION (CLOSED CELL FOAM) REQUIRES 1 TO 2 OPERATORS WITH PLC CONTROLS WEIGHT: 1600 KGS VOLTAGE: 220 VOLTS POWER: 6 KW ULTRASONIC FREQUENCY: 20 kHz GAS PRESSURE: 6 KG PER cm2 QUANTITY: 1 Learn More -
 YY-1170 FACE MASK PACKING MACHINEYY-1170 FACE MASK PACKING MACHINE TYPE: MULTI-FUNCTION PILLOW PACKAGING MACHINE. FUNCTION: FILLING, LABELING, SEALING, WRAPPING BAG FORMATS: PILLOW BAG, FLAT BOTTOM BAG, 3/4 SIDES SEALING BAG, ETC. BAG WIDTH: 30 TO 110mm PRODUCT HEIGHT: MAX 40mm SPEED: 80 TO 120BAG/MIN VOLTAGE AND POWER: 220V/2.4KW DIMENSION (L X W X H): (L)3000 X (W)1350 X (H)1430mm MACHINE WEIGHT: ABOUT 300KG QUANTITY: 1 Learn More
YY-1170 FACE MASK PACKING MACHINEYY-1170 FACE MASK PACKING MACHINE TYPE: MULTI-FUNCTION PILLOW PACKAGING MACHINE. FUNCTION: FILLING, LABELING, SEALING, WRAPPING BAG FORMATS: PILLOW BAG, FLAT BOTTOM BAG, 3/4 SIDES SEALING BAG, ETC. BAG WIDTH: 30 TO 110mm PRODUCT HEIGHT: MAX 40mm SPEED: 80 TO 120BAG/MIN VOLTAGE AND POWER: 220V/2.4KW DIMENSION (L X W X H): (L)3000 X (W)1350 X (H)1430mm MACHINE WEIGHT: ABOUT 300KG QUANTITY: 1 Learn More -
 Z-1613 AUTOMATIC CARTON PACKAGING MACHINEZ-1613 AUTOMATIC CARTON PACKAGING MACHINE CARTONING FORM: HORIZONTAL CARTONING CARTONING SPEED: 30 TO 40 BOXES PER MINUTE (DEPENDING ON CARTON DIMENSIONS) PROCESS REQUIREMENTS: -AUTOMATIC BOX OPENING, CARTONING -GLUE SPRAYING -BOX SEALING VOLTAGE: 220 VOLTS, 60Hz PLC MICRO COMPUTER FULL AUTOMATIC CONTROL MAN-MACHINE INTERFACE OPERATION SYSTEM (HMI), AUTOMATICALLY DISPLAYS THE PACKING SPEED, QUANTITY, FAULT REASON AND OTHER PERFORMANCE PARAMETERS. THE INDENTATION OF THE CARTON IS GENERALLY: 0.4mm DEEP QUANTITY: 1 Learn More
Z-1613 AUTOMATIC CARTON PACKAGING MACHINEZ-1613 AUTOMATIC CARTON PACKAGING MACHINE CARTONING FORM: HORIZONTAL CARTONING CARTONING SPEED: 30 TO 40 BOXES PER MINUTE (DEPENDING ON CARTON DIMENSIONS) PROCESS REQUIREMENTS: -AUTOMATIC BOX OPENING, CARTONING -GLUE SPRAYING -BOX SEALING VOLTAGE: 220 VOLTS, 60Hz PLC MICRO COMPUTER FULL AUTOMATIC CONTROL MAN-MACHINE INTERFACE OPERATION SYSTEM (HMI), AUTOMATICALLY DISPLAYS THE PACKING SPEED, QUANTITY, FAULT REASON AND OTHER PERFORMANCE PARAMETERS. THE INDENTATION OF THE CARTON IS GENERALLY: 0.4mm DEEP QUANTITY: 1 Learn More -
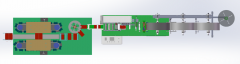 VJ-1116 FULLY AUTOMATIC FLAT FACE MASK PRODUCTION LINE, 300 MASKS PER MINUTEVJ-1116 FULLY AUTOMATIC FLAT FACE MASK PRODUCTION LINE, 300 MASKS PER MINUTE MASK BODY PRODUCTION WITH AUTOMATIC FEEDING TO EAR LOOP WELDING FOR COMPLETE, AUTOMATED PRODUCTION MASK TYPE: SURGICAL, FLAT MASK WITH EAR LOOPS MASK DIMENSIONS: 175 x 95mm CAPACITY: 250 TO 300 PIECES PER MINUTE (FINISHED PRODUCT) NONWOVEN MATERIAL: SPUNBONDED AND MELT-BLOWN FABRIC QUANTITY: 1 Learn More
VJ-1116 FULLY AUTOMATIC FLAT FACE MASK PRODUCTION LINE, 300 MASKS PER MINUTEVJ-1116 FULLY AUTOMATIC FLAT FACE MASK PRODUCTION LINE, 300 MASKS PER MINUTE MASK BODY PRODUCTION WITH AUTOMATIC FEEDING TO EAR LOOP WELDING FOR COMPLETE, AUTOMATED PRODUCTION MASK TYPE: SURGICAL, FLAT MASK WITH EAR LOOPS MASK DIMENSIONS: 175 x 95mm CAPACITY: 250 TO 300 PIECES PER MINUTE (FINISHED PRODUCT) NONWOVEN MATERIAL: SPUNBONDED AND MELT-BLOWN FABRIC QUANTITY: 1 Learn More -
 P-8704 PROTECTIVE SUIT
P-8704 PROTECTIVE SUITREFERENCE NUMBER: P-8704
PROTECTIVE SUIT
MEETS THE TECHNICAL REQUIREMENTS FOR CE AND FDA
HIGH GRAM WEIGHT NONWOVENS OF 68G/M²
ENHANCED WATER RESISTANCE, SYNTHETIC BLOOD PENETRATION RESISTANCE
PREVENT PARTICLES EFFECTIVELY
TYPE 5 AND TYPE 6 PROTECTION
SPE COATING: MOISTURE PROOF, BREATHABLE, FLEXIBLE, NON COMBUSTIBLEHIGH STRENGTH AND TENSILITY
DURABLE AND TEAR RESISTANCE
ANTISTATIC TREATMENT
REDUCE THE ABSORPTION OF HARMFUL SUBSTANCE
SELASTICATED WRISTS, ANKLES AND FACES, HOODED COVERALL
SELF ADHESIVE ZIPPER
CARTON SIZE: 49X45X44mm
GW: 8.50KG
IN A CARTON: 25PCS
QUANTITY: 1
Learn More -
 T-6582 MEDICAL PROTECTIVE SUIT IMPERMEABLE STERILIZED (USED IN INFECTIOUS ISOLATION WARD)
T-6582 MEDICAL PROTECTIVE SUIT IMPERMEABLE STERILIZED (USED IN INFECTIOUS ISOLATION WARD)REFERENCE NUMBER: T-6582
MEDICAL PROTECTIVE SUIT IMPERMEABLE STERILIZED (USED IN INFECTIOUS ISOLATION WARD)
MEDICAL PROTECTIVE SUIT IMPERMEABLE STERILIZED (USED IN INFECTIOUS ISOLATION WARD)
1.NP3030: MEDICAL DISPOSABLE PROTECTIVE CLOTHING,STRONG RESISTANT
2.FABRIC: SF 66 G, COLOR: WHITE RUBBER:CAP/FOOT/CUFF/WAIST FRONT FLAP: DOUBLE-SIDED TAPE, TAPE:BLUE TAPE, ETHYLENE OXIDE DISINFECTION, STERILE
3.PACKAGED: 1PC/BAG, 32PCS/CARTON,10KG/CARTONCARTON SIZE: 470 X300 X420mm/CTN
4.NOTE: CLASS II MEDICAL TREATMENT, SCOPE OF USE: MEDICALPERSONNEL CONTACT WITH POTENTIALLY INFECTIOUS PATIENTSBLOOD, BODY FLUIDS, SECRETIONS, PARTICLES IN THE AIR TOPROVIDE BARRIER, PROTECTION. THIS PRODUCT IS USED FORISOLATION OF OBSERVATION WARD (ROOM), ISOLATION WARD(ROOM), ETC. IT IS STRICTLY PROHIBITED TO USE IN THE ISOLATIONCRITICAL CARE WARD (ROOM) AND OTHER PLACES WITH STRICTCONTROL OF MICROBIAL INDICATORS1PC/BAG, 32PCS/CARTON.
WEIGHT AND DIMENSIONS OF CARTONS: 10KG/CARTON
CARTONS IN A 20 FOOT CONTAINER: 370 BOXESSTERILIZED BY ETHYLENE OXIDE GAS
EACH PIECE IS INDIVIDUALLY VACUUM PACKED
1PER PLASTIC POUCH
QUANTITY: 10,000
Learn More -
 T-6543 MEDICAL UNIVERSAL TENSILE TESTING EQUIPMENT
T-6543 MEDICAL UNIVERSAL TENSILE TESTING EQUIPMENTREFERENCE NUMBER: T-6543
MEDICAL UNIVERSAL TENSILE TESTING EQUIPMENT
SHANDONG INDEPENDENTLY RESEARCH AND DEVELOPMENT THIS COMPREHENSIVE TESTING MACHINE FOR SURGICAL MASK & PROTECTIVE CLOTHING, IT IS WIDELY USED IN A VARIETY OF MASK STRENGTH DETECTION PROJECTS.FEATURES:COMPLY WITH NATIONAL STANDARDS, MEDICAL STANDARDS TESTING REQUIREMENTS, AUTOMATIC SOFTWARE CONTROL SYSTEM, MEET THE REQUIREMENTS OF DATA STORAGE, PRINTING, COMPARISON. THE IMPORTED SERVO MOTOR IS EQUIPPED WITH PRECISION SCREW DRIVE SYSTEM TO ENSURE THE STABILITY OF TEST DATA.
TEST STANDARDS:
GB 19082-2009 TECHNICAL REQUIREMENTS FOR DISPOSABLE PROTECTIVE CLOTHING FOR MEDICAL USE (4.5 BREAKING STRENGTH -THE BREAKING STRENGTH OF MATERIALS IN KEY PARTS OF PROTECTIVE CLOTHING SHOULD NOT BE LESS THAN 45N)
(4.6 ELONGATION AT BREAK -THE ELONGATION AT BREAK OF KEY PARTS OF PROTECTIVE CLOTHING SHOULD NOT BE LESS THAN 15%)
SELF-PRIMING FILTER RESPIRATOR FOR RESPIRATORY PROTECTION ARTICLES5.6.2 BREATHING BONNET -BREATHING BONNET SHALL BE SUBJECT TO AXIAL TENSION"DISPOSABLE MASK: 10N FOR 10S" "REPLACEABLE MASK: 50N FOR 10S"
(5.9 HEADBAND -HEADBAND SHOULD BEAR THE TENSION "DISPOSABLE MASK: 10N, 10S" "REPLACEABLE HALF MASK: 50N FOR 10S" "FULL MASK: 150N FOR 10S")
5.10 JOINTS AND CONNECTING PARTS -JOINTS AND CONNECTING PARTS SHALL BE SUBJECT TO AXIAL TENSION
"REPLACEABLE HALF MASK: 50N FOR 10S" "FULL COVER 250N FOR 10S"
GB/T 32610-2016 TECHNICAL SPECIFICATION FOR DAILY PROTECTIVE MASKS
(6.9 BREAKING STRENGTH OF THE MASK BELT AND THE CONNECTION BETWEEN THE MASK BELT ANDTHE MASK BODY ≥ 20N)
(6.10 FASTNESS TO BREATHING BONNET: NO SLIPPAGE, BREAKAGE OR DEFORMATION SHALL OCCUR)
YY/T 0699-2013 DISPOSABLE SURGICAL MASK
(4.4 MASK BELT -THE BREAKING FORCE AT THE CONNECTION POINT BETWEEN EACH MASK BELT AND THE MASK BODY IS NOT LESS THAN 10N)
YY 0469-2011 SURGICAL MASK FOR MEDICAL USE (5.4.2 MASK BELT)
GB/T 3923.1-1997 DETERMINATION OF BREAKING STRENGTH AND ELONGATION AT BREAK OF FABRICS (STRIP METHOD)
DISPOSABLE RUBBER INSPECTION GLOVES (6.3 TENSILE PROPERTIES)
INSTRUMENT TECHNICAL PARAMETERS:
SPECIFICATION: 200N (STANDARD) 50N, 100N, 500N, 1000N (OPTIONAL)
ACCURACY: BETTER THAN 0.5RESOLUTION OF FORCE VALUE: 0.1N
DEFORMATION RESOLUTION: 0.001mm
TEST SPEED: 0.01mm/MIN ~ 2000mm/MIN (STEPLESS SPEED REGULATION)
WIDTH OF SAMPLE: 30mm (STANDARD FIXTURE) 50mm (OPTIONAL FIXTURE)
CLAMPING OF SAMPLES: MANUAL (PNEUMATIC CLAMPING CAN BE CHANGED)
STROKE: 700mm (STANDARD) 400mm, 1000mm (OPTIONAL)
QUANTITY: 1
Learn More -
 T-6544 SYNTHETIC BLOOD PENETRATION TESTER FOR SURGICAL MASK
T-6544 SYNTHETIC BLOOD PENETRATION TESTER FOR SURGICAL MASKREFERENCE NUMBER: T-6544
SYNTHETIC BLOOD PENETRATION TESTER FOR SURGICAL MASK
TECHNICAL INDICATORS:
1. THE PROTRUDING SAMPLE FIXING DEVICE CAN SIMULATE THE ACTUAL USE STATE OF THE MASK, SET ASIDE THE TEST TARGET AREA WITHOUT DESTROYING THE SAMPLE, AND DISTRIBUTE THE SYNTHETIC BLOOD IN THE TARGET AREA OF THE SAMPLE.
2. THE SPECIAL FIXED-PRESSURE INJECTION DEVICE CAN EJECT A CERTAIN VOLUME OF SYNTHETIC BLOOD IN A CONTROLLED TIME.
3. IT CAN FULLY SIMULATE THE INJECTION SPEED CORRESPONDING TO THE AVERAGE BLOOD PRESSURE OF HUMAN BODY OF 10.6KPA, 16KPA AND 21.3KPA.
4. A FIXED TARGET PLATE IS SET TO BLOCK THE HIGH-PRESSURE EDGE PART OF THE INJECTED LIQUID FLOW AND ONLY ALLOW THE STEADY FLOW PART TO BE SPRAYED ON THE SAMPLE, WHICH INCREASES THE ACCURACY AND REPEATABILITY OF THE LIQUID VELOCITY SPRAYED ON THE SAMPLE.
STANDARDS:
MEDICAL RESPIRATOR TECHNICAL REQUIREMENTS, 5.5 SYNTHETIC BLOOD PENETRATION BARRIER PERFORMANCE
YY/T 0691-2008 INFECTIOUS PATHOGEN PROTECTION EQUIPMENT MEDICAL MASK RESISTANCE TO SYNTHETIC BLOOD PENETRABILITY TEST METHOD (FIXED VOLUME, HORIZONTAL INJECTION)
YY 0469-2011 MEDICAL SURGICAL MASK TECHNOLOGY REQUIRES A BLOOD PENETRATION TEST DEVICE
ISO 22609:2004 INFECTIOUS PATHOGEN PROTECTION EQUIPMENT MEDICAL MASK TEST METHOD FOR RESISTANCE TO SYNTHETIC BLOOD PENETRATION (FIXED VOLUME, HORIZONTAL INJECTION)
ASTM F1862-07 STANDARD TEST METHOD FOR RESISTANCE OF MEDICAL FACE MASKS TO BLOT BY SYNTHETIC BLOOD (HORIZONTAL PROJECTION OF FIXED VOLUME AT A KNOW VELOCITY)
TECHNICAL PARAMETERS:
1. INJECTION DISTANCE: 300mm ~ 305mm ADJUSTABLE
2. NOZZLE DIAMETER: 0.84mm
3. INJECTION SPEED: 450CM/S, 550CM/S, 635CM/S
4. WEIGHT: 35KG
5. POWER SOURCE: AC220V 50HZ
QUANTITY: 1
Learn More -
 T-6547 RESPIRATOR RESISTANCE TESTER FOR FACE MASKS
T-6547 RESPIRATOR RESISTANCE TESTER FOR FACE MASKSREFERENCE NUMBER: T-6547
RESPIRATOR RESISTANCE TESTER FOR FACE MASKS
INTRODUCTION
THE RESPIRATOR RESISTANCE TESTER IS USED TO MEASURE THE INSPIRATORY RESISTANCE AND EXPIRATORY RESISTANCE OF RESPIRATORS AND RESPIRATOR PROTECTORS UNDER SPECIFIED CONDITIONS. IT IS APPLICABLE TO THE NATIONAL LABOR PROTECTION EQUIPMENT INSPECTION INSTITUTIONS, MASK MANUFACTURERS FOR THE GENERAL MASKS, DUST MASKS, MEDICAL MASKS, ANTI-SMOG MASKS PRODUCTS OF THE RELEVANT TESTING AND INSPECTION.
STANDARD
GB 19083-2010 TECHNICAL REQUIREMENTS FOR MEDICAL PROTECTIVE MASKS
GB 2626-2006 RESPIRATOR SELF-SUCTION FILTER RESPIRATOR AGAINST PARTICULATE MATTER
GB/T 32610-2016 TECHNICAL SPECIFICATIONS FOR DAILY PROTECTIVE MASKS
FEATURES:
1. HIGH-DEFINITION LCD DISPLAY.
2. DIGITAL DIFFERENTIAL PRESSURE METER WITH HIGH PRECISION IMPORTED BRAND.
3. HIGH PRECISION IMPORTED BRAND OF DIGITAL FLOWMETER, WITH HIGH FLOW CONTROL ACCURACY FEATURES.
4. THE RESPIRATOR RESISTANCE TESTER CAN SET UP TWO MODES: EXHALATION DETECTION AND INHALATION DETECTION.
5. AUTOMATIC PIPELINE SWITCHING DEVICE OF RESPIRATOR SOLVES THE PROBLEM OF PIPE EXTUBATION AND WRONG CONNECTION WHEN TESTING.
PARAMETER:
1. FLOWMETER RANGE: 0 ~ 100L/MIN, ACCURACY IS 3%
2. DIGITAL PRESSURE DIFFERENCE METER RANGE: 0 ~ 2000PA, ACCURACY: 1PA
3. PUMPING CAPACITY OF AIR PUMP: >100L/MIN
4. OVERALL SIZE: 460 X 1080 X 670mm
5. POWER SOURCE: AC220V 50HZ 650W
6. WEIGHT: 55KG
QUANTITY: 1
Learn More -
 T-6577 PROTECTION SUIT WITH SHOE COVER
T-6577 PROTECTION SUIT WITH SHOE COVERPROTECTION SUIT WITH SHOE COVER
Learn More
HOODED DESIGN
HOODED HEAD DESIGN
ELASTIC DESIGN AT THE MOUTH
CAN ISOLATE DUST AND MICROORGANISMS
SEALED AND PROTECTED BY RUBBER STRIPS
THE WHOLE PIECE DESIGN
EASY TO PUT ON AND TAKE OFF
ELASTIC CUFFS, FLEXIBLE, EFFECTIVE BARRIER, EASIER TO USE
ELASTIC WAISTBAND SLIM ELASTIC WAISTBAND DESIGN, CLOSE THE WAIST, WORK MORE FREELY
CERTIFICATE: CE
MOQ: 1000PCS
MODEL: CONJOINED SPECS: S/160, M/165, L/170, XL/175, XXL/180 ALL SIZES ARE AVAILABLE, PRICES ARE SAME
TYPE:
NON-STERILE: 9.5KG/BOX, 30PCS/BOX, 560 X 430 X 360mm
STERILIZED FOR HOSPITAL: 13KG/BOX, 40PCS/BOX, 600 X 400 X 450mm
PRODUCT DESCRIPTION: CLOTHING MADE FROM FABRICS WITH ISOLATION FUNCTION SUCH AS VIRUS-CONTAINING AEROSOLS AND VIRUS-CONTAINING LIQUIDS. WHEN TAKEN OFF, THE OUTER SURFACE OF THE PROTECTIVE CLOTHING SHOULD NOT COME IN CONTACT WITH THE HUMAN BODY. THIS PRODUCT DOES NOT HAVE FLAME RETARDANT PROPERTIES.
INTENDED USE: OCCUPATIONAL PROTECTIVE CLOTHING FOR MEDICAL PERSONNEL IN MEDICAL INSTITUTIONS TO PREVENT THE TRANSMISSION OF VIRUSES FROM PATIENTS TO THE MEDICAL PERSONNEL WITH AIR OR LIQUID.
