Search results for: 'Euro'
- Related search terms
- Euromac
- Euromec
- Eurome
- euro str
- euro 1 2 3 4 5
-
 TT-1174 DUSENBERY SLITTER REWINDER, YEAR 2000, WORKING WIDTH 2050mmTT-1174 DUSENBERY SLITTER REWINDER, YEAR 2000, WORKING WIDTH 2050mm GENERAL BRAND: DUSENBERY EUROPE LIMITED MODEL: 281 YEAR: 2000 SPEED: FOIL MAX. 400 M/MIN CARDBOARD MAX. 70 M/MIN QUANTITY: 1 Learn More
TT-1174 DUSENBERY SLITTER REWINDER, YEAR 2000, WORKING WIDTH 2050mmTT-1174 DUSENBERY SLITTER REWINDER, YEAR 2000, WORKING WIDTH 2050mm GENERAL BRAND: DUSENBERY EUROPE LIMITED MODEL: 281 YEAR: 2000 SPEED: FOIL MAX. 400 M/MIN CARDBOARD MAX. 70 M/MIN QUANTITY: 1 Learn More -
 YY-2051 NEW CASHMERE AND SHEEP WOOL CARDING MACHINE, PRODUCTION EFFICIENCY 98%YY-2051 NEW CASHMERE AND SHEEP WOOL CARDING MACHINE, PRODUCTION EFFICIENCY 98% DETAILS: CONDITION: NEW APPLICABLE INDUSTRIES: MANUFACTURING PLANT, FARMS CORE COMPONENTS: BEARING APPLICATION: WOOL, CASHMERE AUTOMATIC GRADE: AUTOMATIC PRODUCTION EFFICIENCY: 98% VOLTAGE: 220 VOLTS, 3-PHASE OR AS PER CUSTOMER REQUIREMENT DIMENSIONS (L X W X H): 3000 X 2400 X 1800(mm) WEIGHT: 5800 KG QUANTITY: 1 Learn More
YY-2051 NEW CASHMERE AND SHEEP WOOL CARDING MACHINE, PRODUCTION EFFICIENCY 98%YY-2051 NEW CASHMERE AND SHEEP WOOL CARDING MACHINE, PRODUCTION EFFICIENCY 98% DETAILS: CONDITION: NEW APPLICABLE INDUSTRIES: MANUFACTURING PLANT, FARMS CORE COMPONENTS: BEARING APPLICATION: WOOL, CASHMERE AUTOMATIC GRADE: AUTOMATIC PRODUCTION EFFICIENCY: 98% VOLTAGE: 220 VOLTS, 3-PHASE OR AS PER CUSTOMER REQUIREMENT DIMENSIONS (L X W X H): 3000 X 2400 X 1800(mm) WEIGHT: 5800 KG QUANTITY: 1 Learn More -
 T-6965 COMPLETE FILLING LINE FOR WATER AND CSD IN PET BOTTLES, CAPACITY 16,000 BPHT-6965 COMPLETE FILLING LINE FOR WATER AND CSD IN PET BOTTLES, CAPACITY 16,000 BPH NOMINAL PRODUCTION: 16,000 BPH BOTTLE SIZES: 0.5 / 1 LT USED FOR FILLING: STILL AND CARBONATED WATER / CSD CONDITIONS: GOOD, REGULARLY SERVICED Learn More
T-6965 COMPLETE FILLING LINE FOR WATER AND CSD IN PET BOTTLES, CAPACITY 16,000 BPHT-6965 COMPLETE FILLING LINE FOR WATER AND CSD IN PET BOTTLES, CAPACITY 16,000 BPH NOMINAL PRODUCTION: 16,000 BPH BOTTLE SIZES: 0.5 / 1 LT USED FOR FILLING: STILL AND CARBONATED WATER / CSD CONDITIONS: GOOD, REGULARLY SERVICED Learn More -
 CEMENT PLANT 1.0 MIO TONS PER YEARREFERENCE NUMBER: T-6943 CEMENT PLANT 1.0 MIO TONS PER YEAR CEMENT PLANT 1.0 MIO TONS PER YEAR FIRST CLASS EUROPEAN MACHINES POWER STATION COAL-FIRED, FUEL OR GAS INSTALLED, NEVER STARTED UP, NEW / UNUSED EXCELLENT CONDITION LETTER OF INTENT (LOI) REQUIRED QUANTITY: 1 Learn More
CEMENT PLANT 1.0 MIO TONS PER YEARREFERENCE NUMBER: T-6943 CEMENT PLANT 1.0 MIO TONS PER YEAR CEMENT PLANT 1.0 MIO TONS PER YEAR FIRST CLASS EUROPEAN MACHINES POWER STATION COAL-FIRED, FUEL OR GAS INSTALLED, NEVER STARTED UP, NEW / UNUSED EXCELLENT CONDITION LETTER OF INTENT (LOI) REQUIRED QUANTITY: 1 Learn More -
 LAROCHE TEARING MACHINE, WORKING WIDTH 1400mmREFERENCE NUMBER: T-6910 LAROCHE TEARING MACHINE, WORKING WIDTH 1400mm LAROCHE TEARING MACHINE TYPE SUPER EUROP WORKING WIDTH 1400mm 1 CYLINDER Ø 1000mm MANUAL FEEDING MOTOR 220/380 V 60 HP 45 KW FILTERS 1 SPARE CYLINDER QUANTITY: 1 Learn More
LAROCHE TEARING MACHINE, WORKING WIDTH 1400mmREFERENCE NUMBER: T-6910 LAROCHE TEARING MACHINE, WORKING WIDTH 1400mm LAROCHE TEARING MACHINE TYPE SUPER EUROP WORKING WIDTH 1400mm 1 CYLINDER Ø 1000mm MANUAL FEEDING MOTOR 220/380 V 60 HP 45 KW FILTERS 1 SPARE CYLINDER QUANTITY: 1 Learn More -
 BACTERIAL FILTRATION UNITREFERENCE NUMBER: Y-2078 BACTERIAL FILTRATION UNIT NOT ONLY CONFORMS TO THE REQUIREMENTS OF MEDICAL SURGICAL MASK TECHNOLOGY IN THE TEST METHOD FOR FILTER EFFICIENCY (BFE) BACTERIA FIRST TEST INSTRUMENT, BUT ALSO CONFORMS TO THE AMERICAN SOCIETY FOR TESTING MATERIAL, THE REQUIREMENTS OF THE EUROPEAN EN14683 STANDARDS, INNOVATIVE IMPROVEMENTS WERE MADE ON THE BASIS OF THIS, WITH DOUBLE PNEUMATIC CONTRAST SAMPLING METHOD AT THE SAME TIME, IMPROVE THE PRECISION OF SAMPLING. IT IS SUITABLE FOR MEASURING AND TESTING DEPARTMENTS, SCIENCE RESEARCH INSTITUTE, MASK MANUFACTURERS AND OTHER RELEVANT DEPARTMENTS TO TEST THE PERFORMANCE OF MASK BACTERIAL FILTRATION EFFICIENCY. PRODUCT FEATURES: NEGATIVE PRESSURE TEST SYSTEM TO ENSURE THE SAFETY OF OPERATORS BUILT-IN PERISTALTIC PUMP IN NEGATIVE PRESSURE CABINET THE PERISTALTIC PUMP FLOW CAN BE SET THE FLOW RATE OF NIGHT SPRAY CAN BE SET AND THE ATOMIZATION EFFECT IS GOOD EMBEDDED HIGH SPEED INDUSTRIAL MICROCOMPUTER CONTROL 10.4 INCHES INDUSTRIAL HIGH BRIGHTNESS COLOR TOUCH SCREEN USB INTERFACE, SUPPORT U DISK DATA STORAGE CABINET BUILT-IN HIGH BRIGHTNESS LIGHTS BUILT-IN LEAKAGE PROTECTION SWITCHES TO PROTECT THE SAFETY OF OPERATORS THE INNER LAYER OF THE CABINET IS MADE OF STAINLESS STEEL, THE OUTER LAYER IS SPRAYED WITH PLASTIC AND COLD-ROLLED, AND THE INNER AND OUTER LAYERS ARE INSULATED AND FLAME RETARDANT. THE FRONT SWITCH TYPE GLASS DOOR IS CONVENIENT FOR THE EXPERIMENTER TO OBSERVE AND OPERATE. DETACHABLE BRACKET, ADJUSTABLE BRACKET HEIGHT SUPPORTING AND MOVING DUAL PURPOSE CASTERS SPECIFICATIONS: A ROUTE SAMPLING FLOW: 28.3L PER MINUTE B ROUTE SAMPLING FLOW: 20.3L PER MINUTE SPRAY FLOW: 8 TO 10L PER MINUTE PERISTALTIC PUMP FLOW: 0.006 TO 3.0 mL PER MINUTE FRONT PRESSURE OF A ROUTE SAMPLING FLOW METER: (-20 TO 0) kPa FRONT PRESSURE OF B ROUTE SAMPLING FLOW METER: (-20 TO 0) kPa FRONT PRESSURE OF SPRAY FLOW METER: (0 TO 300) kPa AMBIENT TEMPERATURE: (-40 TO 99) ℃ NEGATIVE PRESSURE OF THE AEROSOL CHAMBER: (-90 TO 120) kPa QUANTITY: 1 Learn More
BACTERIAL FILTRATION UNITREFERENCE NUMBER: Y-2078 BACTERIAL FILTRATION UNIT NOT ONLY CONFORMS TO THE REQUIREMENTS OF MEDICAL SURGICAL MASK TECHNOLOGY IN THE TEST METHOD FOR FILTER EFFICIENCY (BFE) BACTERIA FIRST TEST INSTRUMENT, BUT ALSO CONFORMS TO THE AMERICAN SOCIETY FOR TESTING MATERIAL, THE REQUIREMENTS OF THE EUROPEAN EN14683 STANDARDS, INNOVATIVE IMPROVEMENTS WERE MADE ON THE BASIS OF THIS, WITH DOUBLE PNEUMATIC CONTRAST SAMPLING METHOD AT THE SAME TIME, IMPROVE THE PRECISION OF SAMPLING. IT IS SUITABLE FOR MEASURING AND TESTING DEPARTMENTS, SCIENCE RESEARCH INSTITUTE, MASK MANUFACTURERS AND OTHER RELEVANT DEPARTMENTS TO TEST THE PERFORMANCE OF MASK BACTERIAL FILTRATION EFFICIENCY. PRODUCT FEATURES: NEGATIVE PRESSURE TEST SYSTEM TO ENSURE THE SAFETY OF OPERATORS BUILT-IN PERISTALTIC PUMP IN NEGATIVE PRESSURE CABINET THE PERISTALTIC PUMP FLOW CAN BE SET THE FLOW RATE OF NIGHT SPRAY CAN BE SET AND THE ATOMIZATION EFFECT IS GOOD EMBEDDED HIGH SPEED INDUSTRIAL MICROCOMPUTER CONTROL 10.4 INCHES INDUSTRIAL HIGH BRIGHTNESS COLOR TOUCH SCREEN USB INTERFACE, SUPPORT U DISK DATA STORAGE CABINET BUILT-IN HIGH BRIGHTNESS LIGHTS BUILT-IN LEAKAGE PROTECTION SWITCHES TO PROTECT THE SAFETY OF OPERATORS THE INNER LAYER OF THE CABINET IS MADE OF STAINLESS STEEL, THE OUTER LAYER IS SPRAYED WITH PLASTIC AND COLD-ROLLED, AND THE INNER AND OUTER LAYERS ARE INSULATED AND FLAME RETARDANT. THE FRONT SWITCH TYPE GLASS DOOR IS CONVENIENT FOR THE EXPERIMENTER TO OBSERVE AND OPERATE. DETACHABLE BRACKET, ADJUSTABLE BRACKET HEIGHT SUPPORTING AND MOVING DUAL PURPOSE CASTERS SPECIFICATIONS: A ROUTE SAMPLING FLOW: 28.3L PER MINUTE B ROUTE SAMPLING FLOW: 20.3L PER MINUTE SPRAY FLOW: 8 TO 10L PER MINUTE PERISTALTIC PUMP FLOW: 0.006 TO 3.0 mL PER MINUTE FRONT PRESSURE OF A ROUTE SAMPLING FLOW METER: (-20 TO 0) kPa FRONT PRESSURE OF B ROUTE SAMPLING FLOW METER: (-20 TO 0) kPa FRONT PRESSURE OF SPRAY FLOW METER: (0 TO 300) kPa AMBIENT TEMPERATURE: (-40 TO 99) ℃ NEGATIVE PRESSURE OF THE AEROSOL CHAMBER: (-90 TO 120) kPa QUANTITY: 1 Learn More -
 T-6545 MASK BACTERIAL FILTRATION EFFICIENCY (BFE) DETECTOR TESTER
T-6545 MASK BACTERIAL FILTRATION EFFICIENCY (BFE) DETECTOR TESTERREFERENCE NUMBER: T-6545
MASK BACTERIAL FILTRATION EFFICIENCY (BFE) DETECTOR TESTER
PRODUCT INTRODUCTION:
MASK BACTERIA FILTRATION EFFICIENCY KEY PERFORMANCE INDICATORS (BFE) DETECTOR TESTER NOT ONLY CONFORMS TO THE REQUIREMENTS OF MEDICAL SURGICAL MASKS TECHNOLOGY YY0469-2011 APPENDIX B IN THE TEST METHOD FOR FILTER EFFICIENCY (BFE) BACTERIA FIRST B. 1.1.1 TEST INSTRUMENT, BUT ALSO CONFORMS TO THE AMERICAN SOCIETY FOR TESTING MATERIAL ASTMF2100, ASTMF2101, THE REQUIREMENTS OF THE EUROPEAN EN14683 STANDARDS, INNOVATIVE IMPROVEMENTS WERE MADE ON THE BASIS OF THIS, WITH DOUBLE PNEUMATIC CONTRAST SAMPLING METHOD AT THE SAME TIME, IMPROVE THE PRECISION OF SAMPLING,IT IS SUITABLE FOR MEASURING AND TESTING DEPARTMENTS, SCIENTIFIC RESEARCH INSTITUTES, MASK MANUFACTURERS AND OTHER RELEVANT DEPARTMENTS TO TEST THE PERFORMANCE OF MASK BACTERIAL FILTRATION EFFICIENCY.
EXECUTION STANDARD:
Q/0212 ZRB003-2015 MEDICAL SURGICAL MASK
BACTERIAL FILTRATION EFFICIENCY (BFE) DETECTOR RELATED INTELLECTUAL PROPERTY:
NO.: ZL200820224142.6 ELECTRIC FLOW CONTROL VALVE
NO.: ZL200820224143.0 GAS CAPACITY
NO.: ZL200920308391.8 AIR FILTRATIONMATERIAL FILTRATION EFFICIENCY DETECTOR
PRODUCT FEATURES:
NEGATIVE PRESSURE TEST SYSTEM TO ENSURE THE SAFETY OF OPERATORS;
BUILT -IN PERISTALTIC PUMP IN NEGATIVE PRESSURE CABINET, A, B TWO -WAY, SIX ANDERSEN; THE PERISTALTIC PUMP FLOW CAN BE SET;
THE FLOW RATE OF NIGHT SPRAY CAN BE SET AND THE ATOMIZATION EFFECT IS GOOD.
EMBEDDED HIGH SPEED INDUSTRIAL MICROCOMPUTER CONTROL; 10.4 INCHES INDUSTRIAL HIGH BRIGHTNESS COLOR TOUCH SCREEN;
USB INTERFACE, SUPPORT U DISK DATA STORAGE;
CABINET BUILT-IN HIGH BRIGHTNESS LIGHTS; BUILT-IN LEAKAGE PROTECTION SWITCH TO PROTECT THE SAFETY OF OPERATORS;
THE INNER LAYER OF THE CABINET IS MADE OF STAINLESS STEEL, THE OUTER LAYER IS SPRAYED WITH PLASTIC AND COLD-ROLLED, AND THE INNER AND OUTER LAYERS ARE INSULATED AND FLAME RETARDANT.
THE FRONT SWITCH TYPE GLASS DOOR IS CONVENIENT FOR THE EXPERIMENTER TO OBSERVE AND OPERATE.
DETACHABLE BRACKET, ADJUSTABLE BRACKET HEIGHT; SUPPORTING AND MOVING DUAL PURPOSE CASTERS.
QUANTITY: 1
Learn More -
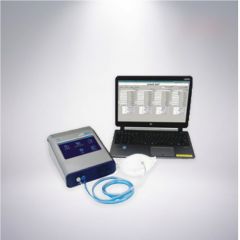 T-6552 MASK AIR TIGHTNESS TESTER
T-6552 MASK AIR TIGHTNESS TESTERREFERENCE NUMBER: T-6552
MASK AIR TIGHTNESS TESTER
MASK AIR TIGHTNESS TESTER
HOW TO CHOOSE A HIGH-QUALITY MASK NOT ONLY DEPENDS ON THE FILTERING EFFICIENCY OF THEMASK, BUT ALSO TO CONFIRM WHETHER THE MASK IS COMPLETELY IN CONTACT WITH THE FACE, OTHERWISE AEROSOL PARTICLES SUCH AS GERMS THAT HAVE NOT BEEN FILTERED BY THE MASK WILL BE INHALED FROM THE INADEQUATE ADHESION. THEREFORE, IT IS VERY IMPORTANT TO CHECK THE WEARING STATUS OF THE MASK. THE EUROPEAN AND AMERICAN COUNTRIES HAVE LISTED THE APPLICATION TEST OF THE MASK AS A STRONG INSPECTION ITEM.
FOR EXAMPLE, THE N95 MASK IS ONE OF 9 TYPES OF PARTICULATE PROTECTIVE MASKS CERTIFIED BY NIOSH (NATIONAL INSTITUTE OF OCCUPATIONAL SAFETY AND HEALTH). "N" INDICATES OIL RESISTANCE."95" INDICATES THAT THE PARTICLE CONCENTRATION IN THE MASK IS MORE THAN 95% LOWER THAN THAT OF THE PARTICLES OUTSIDE THE MASK WHEN EXPOSED TO THE SPECIFIED NUMBER OF SPECIAL TEST PARTICLES. N95 IS NOT A SPECIFIC PRODUCT NAME, AS LONG AS IT MEETS THE N95 STANDARD AND PASSES THE NIOSH REVIEW, IT CAN BE CALLED "N95 MASK".
N95 TYPE MASKS, IN ADDITION TO THE FILTERING EFFICIENCY OF THE MASKS, THE CLOSENESS OF THE MASKS TO THE FACE IS ONE OF THE IMPORTANT FACTORS THAT DETERMINE THE EFFECTIVENESS OF THE USE OF THE MASKS. THE SUITABILITY OF DIFFERENT TYPES OF MASKS FOR THE HUMAN FACE VARIES GREATLY.
THEREFORE, BEFORE USING A MASK, THE SUITABILITY OF THE MASK SHOULD BE CHECKED FIRST. DURING THETIGHTNESS TEST OF THE WEARER'S FACE, ENSURE THAT AIR CAN PASS IN AND OUT THROUGH THE MASK WHEN IT IS CLOSE TO THE EDGE OF THE FACE. THE MASK TIGHTNESS TESTER CAN QUICKLY COMPLETE THE TIGHTNESS TEST OF RESPIRATORS SUCH AS MASKS TO ENSURE THAT IT PROVIDES GOOD PROTECTION PERFORMANCE. IT CONFORMS TO THE CHINESE RESPIRATOR STANDARD GB2626-2019 STANDARD, OSHA / CSA STANDARD AND CHINA GENERAL ADMINISTRATION OF QUALITY SUPERVISION, INSPECTION AND QUARANTINE "GB 19083-2010 TECHNICAL REQUIREMENTS FOR MEDICAL PROTECTIVE MASKS" JOINTLY ISSUED BY THE CHINA NATIONAL STANDARDIZATION ADMINISTRATION COMMITTEE.
ADHESION (SUITABILITY TEST): THE MASK DESIGN SHOULD PROVIDE GOOD ADHESION. THE OVERALL FIT FACTOR OF THE MASK SHOULD NOT BE LESS THAN 100. THIS TEST THE REQUIREMENTS WERE FORMALLY IMPLEMENTED ON AUGUST 1, 2011.
Learn MoreMASK TIGHTNESS TESTER USES CNC TECHNOLOGY, SUITABLE FOR 100 / 99 / P3 / HEPASERIES MASKS DISPOSABLE FILTER MASK TIGHTNESS TEST (INCLUDING DISPOSABLE DUST MASKS SUCH ASN95 / N90 / KN95), GAS MASKS / BREATHING MASKS, ADHESIVENESS TEST OF HALF-MASK AND FULL FACE MASK, INDEPENDENT OR COMPUTER CONTROL, FIVE LANGUAGES SWITCH DISPLAY, EQUIPPED WITH MULTIPLE COMMUNICATION INTERFACES (USB, ETHERNET), WIFI CAN ALSO BE ENABLED, ONE COMPUTERCAN CONTROL FOUR INSTRUMENTS AT THE SAME TIME.PARAMETER:CONCENTRATION RANGE: 0 ~ 100,000 / CM3GRAIN DIAMETER: 0.02 ~ 1.0^MFLOW: SAMPLING FLOW: 100CM3 / MINTOTAL FLOW: 700CM3 / MINCLOSENESS COEFFICIENT TEST: DIRECT TEST (COUT/ CIN)LIQUOR: 99.5% + ISOPROPANOL (ANALYTICAL GRADE)DISPLAY: 7INCH TRUE COLOR TOUCH SCREENCOMMUNICATION INTERFACE: USB X 3 (HOST X 2,DEVICE X 1) ETHERNET INTERFACE X1CONNECTION PORT: ENVIRONMENT PORT, SAMPLING PORTWIFI: EQUIPPEDLANGUAGES: ENGLISH, FRENCH, SPANISH, PORTUGUESE, CHINESEFLOW CONTROL: SENSOR CONTROLPC CONTROLLABLE OPERATION: ONE COMPUTER CAN CONTROL 4 INSTRUMENTS AT THE SAME TIMEDATA OUTPUT FORMAT: MICROSOFT EXCELWORKING TEMPERATURE: 15 ~ 35 °CPOWER SOURCE: AC 110 —240V 50 / 60HZAPPEARANCE SIZE: 208 X 117 X 262MMWEIGHT: 2.1KGACCESSORIES: ALCOHOL REAGENT BOTTLE, PROTECTIVE CAP, REAGENT STICK, ZERO-COUNT FILTER, STRAINER, SAMPLING TUBE, INSTRUCTION MANUAL, AC ADAPTER, TOUCH SCREEN PEN, OPTIONAL COMPUTER.OPTIONAL: TEST KIT FOR TIGHTNESS COEFFICIENTIN ORDER TO PREVENT INFECTION AT THE MEDICAL SITE AND DURING LABOR WORK, WORKERS MUST BE PROTECTED FROM INHALABLE HAZARDOUS SUBSTANCES IN THE WORKPLACE, AND RESPIRATORS SUCH AS MASKS MUST BE WORN DURING WORK.SELECT A RESPIRATOR, SUCH AS A MASK, BASED ON YOUR FACIAL FEATURES, AND EVALUATE THE TIGHTNESS BETWEEN THE RESPIRATOR, SUCH AS A MASK AND THE FACE, TO CHECK WHETHER THERE ARE GAPS OR LEAKS THAT PUT WORKERS AT RISK. THE MASK TIGHTNESS TESTER CAN QUICKLY COMPLETE THE TIGHTNESS TEST OF RESPIRATORS SUCH AS MASKS TO ENSURE THAT IT PROVIDES GOOD PROTECTIVE PERFORMANCE. SECURITY EXPERTS WILL ALSO DEVELOP PROTECTION SCHEMES AND STANDARD REGULATIONS BASED ON THE RESULTS OF THE TIGHTNESS TEST. WIDELY USED IN HOSPITALS, MANUFACTURING PLANTS, PRODUCTION SITES, FIREFIGHTING WORKPLACES AND OTHER OFFICIAL TESTING AGENCIES.MASK TIGHTNESS TESTER FEATURES:• QUANTITATIVE TIGHTNESS TEST FOR RESPIRATORS SUCH AS MASKS (QNFT)• APPLICABLE TO 100/99 / P3 / HEPA SERIES MASK DISPOSABLE FILTER MASK TIGHTNESS TEST (INCLUDING N95 / N90 / KN95 AND OTHER DISPOSABLE DUST MASKS)• ADHESION TEST OF HALF MASK AND FULL FACE MASK• GAS MASK TIGHTNESS TEST• PAPR MASK TIGHTNESS TEST• SCBA RESPIRATOR MASK TIGHTNESS TEST• 7INCH TRUE COLOR TOUCH SCREEN• INDEPENDENT OR COMPUTER CONTROLLED• USING CNC TECHNOLOGY• ENGLISH, FRENCH, SPANISH, PORTUGUESE, CHINESE LANGUAGE SWITCHING DISPLAY• COMPLIES WITH US OSHA STANDARDS, CANADIAN STANDARDS ASSOCIATION (CSA) GUIDELINES, INCLUDING N95• EQUIPPED WITH A VARIETY OF COMMUNICATION INTERFACES (USB, ETHERNET), AND CAN ALSO ENABLE WIFI• ONE COMPUTER CAN CONTROL FOUR INSTRUMENTS AT THE SAME TIMEQUANTITY: 1 -
 D-2380 AUTOMATED FILTER TESTER
D-2380 AUTOMATED FILTER TESTERTHE AUTOMATED FILTER TESTER MODEL 9981 CONTINUES TO BE THE BEST SOLUTION FOR TESTING PARTICULATE RESPIRATOR FILTERS, DISPOSABLE FILTERING FACE PIECES, AND A WIDE ASSORTMENT OF FILTER MEDIA. OUR FILTER TESTERS HAVE BEEN USED IN QUALITY CONTROL AND MANUFACTURING BY LEADING FILTER AND FILTER MEDIA MANUFACTURERS AND REFERENCE TEST INSTITUTES AROUND THE WORLD FOR MORE THAN 20 YEARS DUE TO THEIR PROVEN DURABILITY AND RELIABILITY, WHICH IS VALUED IN DEMANDING MANUFACTURING ENVIRONMENTS AND QA/QC LABORATORIES.
THIS MODEL OFFERS HIGHER SENSITIVITY AND RESOLUTION THAN PREVIOUS MODELS - UP TO 99.9999 PERCENT EFFICIENCY HAS BEEN PRODUCED WITH EMERY OIL AT CONCENTRATIONS OF 200 MG/M³. THE NEW SERVICEABLE PHOTOMETERS ARE LOWERING THE COST OF OWNERSHIP AND ENABLE PROACTIVE SCHEDULING OF MAINTENANCE FOR MINIMAL IMPACT TO PRODUCTION SCHEDULES.
THE MODEL 9981 FEATURES A HIGH DEGREE OF AUTOMATION AND SELF-DIAGNOSTICS THAT GREATLY SIMPLIFIES OPERATION, INCREASES THROUGHPUT, AND IMPROVES OVERALL MEASUREMENT PERFORMANCE. WHEN LOOKING FOR A STAND-ALONE TESTER TO DETERMINE PENETRATION OR FILTER EFFICIENCY AND PRESSURE DROP OF YOUR MEDIA, FILTER CARTRIDGES, FILTERS, AND RESPIRATORY MASKS THE MODEL 9981 IS UP TO THE CHALLENGE.
THE BUILT-IN CAPABILITY TO TEST WITH SALT AND OIL MEANS THAT JUST ONE UNIT IS NEEDED TO TEST YOUR PRODUCT TO:
• US 42 CFR 84, GB2626, JMOL
• ISO 16900-3, EN 143
• ISO 23328-1 (AND MORE)
APPLICATIONS
• USA COMMERCIAL RESPIRATOR REGULATION 42 CFR PART 84
• CHINESE RESPIRATOR STANDARD GB2626
• JAPANESE RESPIRATOR STANDARD JMOL
• EUROPEAN EN 143, ISO 16900-3 AND RELATED RESPIRATOR STANDARDS
• ISO 23328-1 (TESTING FILTERS FOR ANAESTHETIC USE)
• FILTER MEDIA TESTING
• CONSULT OUR REPRESENTATIVE FOR MORE DETAILS
INCLUDED ITEMS
• REFERENCE MEDIA SHEETS
• FLAT-MEDIA AND GRAVIMETRIC FILTER HOLDER
• REFERENCE PRESSURE DROP PLATE
• OIL AND SALT AEROSOL GENERATORS
• SELECTED SPARE PARTS
• CONTACT US FOR CUSTOM FILTER HOLDERSEMAIL US FOR PRICE & PICTURES
Learn More
INCLUDE OUR REFERENCE NUMBER -
 How To Choose an N95 Respirator Mask For Coronavirus Prevention? All You Need to Know Before Buying Face Masks
How To Choose an N95 Respirator Mask For Coronavirus Prevention? All You Need to Know Before Buying Face MasksHow To Choose an N95 Respirator Mask For Coronavirus Prevention? All You Need to Know Before Buying Face Masks.
1. What’s an N95 mask?
An N95 respirator is a respiratory protective face mask, designed to help reduce the user’s exposure to airborne particles including very small particles (0.3 microns) and large droplets. N95 respirator face mask literally has a filtration efficiency of at least 95% against non-oily particles.
2. Differences between N95 mask and surgical mask?
Generally, the N95mask also includes an N95 surgical mask. So, here we will just compare a standard N95 particulate mask with a disposable surgical mask. Surgical masks are designed to be worn by healthcare professionals during surgery and nursing, to help prevent contamination of the surgical field or the patient by capturing liquid droplets that are expelled by the user.
An N95 particulate mask blocks at least 95 percent of very small (0.3 microns) test particles, and while a disposable surgical mask is fluid-resistant and protects the user against large particles (5 microns), droplets, sprays or splatter.
Different from a loose-fitting, disposable surgical mask, an N5 mask is tight-fitting to achieve a very close facial fit, which guarantees a minimal leakage around the edges of the mask when the user inhales. If properly fitted, the filtration capabilities of N95 respirators exceed those of disposable surgical face masks.Speaking of the N95 mask price and disposable surgical mask price, the N95 respirator mask is pricier than a surgical mask. In contrast, the N95 mask is reusable but the common surgical mask is disposable. Strictly speaking, N95 respirator masks should be worn for a maximum of eight hours while the disposable surgical mask is recommended to be replaced every four hours.
However, during the novel coronavirus outbreak, face masks are in short supply and everyone discards one or two face masks every day. A surgical mask could be used for a whole day in a good condition of no damage, no moisture, and no deformation. According to some researches, the N95 respirator mask used for two days still maintains a filtration efficiency of at least 95%, while the filtration efficiency of an N95 respirator used for three days reduced to 94.7%. So, if an N95 respirator is in good maintenance, you can wear it for at least 2 days, a week, maybe even a month.
3. Types of N95 mask
Generally, according to their intended use, the types of N95 masks include the standard N95 particulate respirators and N95 surgical respirator masks. Additionally, there are N95 masks with a breathing valve or not.
1) Differences between standard N95 particulate respirator and N95 surgical mask:
How to differentiate standard N95 particulate-filtering respirators and N95 surgical masks? The N95 surgical mask is literally a combination of N95 respirator and surgical mask, it is both certificated by NIOSH as an N95 respirator and also cleared by the FDA as a surgical mask. N95 surgical respirator masks are also referred to as medical respirators, healthcare respirators, or surgical N95s.
The significant difference between a standard N95 particulate respirator and an N95 surgical mask is that the N95 surgical mask is fluid-resistant while the standard N95 particulate respirator is not.
An N95 particulate respirator, also called N95 dust mask or N95 pollution mask, helps reduce very small particles inhaled by the user, and it is used for respiratory protection when the user might be exposed to particulate hazards. With a fluid resistance plus, an N95 surgical mask help reduce particles both inhaled and exhaled by the user, it is designed to be used during surgery and nursing tasks, whatever the user requires respiratory protection, expelled particulates or requires fluid resistance.
2) Differences between N95 masks with a breathing valve or not:
Many people question: Is an N95 mask with an exhalation valve better than the one without a valve?
In fact, the N95 respirators with an exhalation valve are not recommended to be used for coronavirus prevention, because the valve is a one-way vent that couldn’t keep your exhalation to yourself. It means that this valved N95 mask makes your breathing out easier and helps reduce heat build-up to avoid fogging up your glasses, however, it’s not that friendly to others.
Besides, the N95 mask with the valve is pricier than the one without a valve.4. What are the differences between N95, KN95, FFP2, and KF94?
When you search for the N95 respirators, there are many respirators such as KN95, FFP2, and KF94 that appeared in the same breath. What’s different between these respirators of different letters?
Actually, both the N95, KN95, FFP2 and KF94 respirators have almost the same filtration efficiency, it means that KN95, FFP2, and KF94 are as effective as the N95 respirator mask. The main difference between these respirators is that they are tested by different nations.
The N95 mask has a full name of NIOSH-certified N95 respirator, which means these respirators are tested of 95% filtration and certified by the U.S. National Institute of Occupational Safety and Health (NIOSH). The KN95 respirator is a particulate-filtering mask tested using the China criteria. The FFP2 is a filtering facepiece score of 94% filter capacity approved by Europe, and the KF94 is tested by Korea criteria.
To simply put, we could put these respirators into an equation as below:
N100 (99.97%) = FFP3 (99.95%) > N95 (95%) = KN95 (95%) = FFP2 (94%) = KF94 (94%) > KN90 (90%).
Learn More
Anti Virus Flu Mask Meets FFP2 N95 KN95 KF94 Guidance
